Protein, Part 1 – Definitions and Technical Background
by Lyle McDonald
If you ask 10 bodybuilders what the most important nutrient is for putting on muscle, you will most likely get 10 identical answers (a rarity in sports nutrition). That answer is, of course: PROTEIN! Every bodybuilder knows that, no matter what else you do, if you aren’t getting enough protein, you won’t grow.
Unfortunately, the obsession that bodybuilders have with protein has made them susceptible to all kinds of marketing hype. Whether it involves protein intake, quality, type, form, etc., marketing types know how to push a bodybuilder’s buttons when it comes to protein.
If you want to sell a lot of protein powder, just throw around terms like ‘nitrogen balance/retention’, ‘biological value’ or ‘anti-catabolic’ and the money will start rolling in. If you want to scare bodybuilders into listening to you, start talking about amino acid oxidation (burning) with high protein intakes and how ‘horrible’ it is (too bad sarcasm doesn’t carry to the written word). Then move in for the kill and sell them your product.
Like most aspects of bodybuilding (and the supplement industry in general), the issue of protein is driven more by marketing hype than physiological reality. The purpose of this article series is to address some technical issues regarding protein and to clear up some of the major misconceptions that currently exist. Although some of the information is decidedly technical, I will try to avoid unnecessarily nasty details as much as possible.
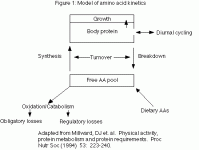
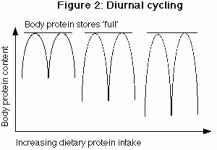
This first part will deal primarily with some definitions and technical background, so that later information will make more sense. In part 2, protein quality as well as a simplified model of amino acid metabolism will be developed. This will help readers to understand the discussion of adaptation to both high and low protein intake, as well as varying calorie levels. Although a discussion of every amino acid is beyond the scope of this article series, specific amino acids such as glutamine, leucine and alanine will be discussed as necessary.
Section 1: Definitions and basic background
What is a protein?
Proteins are organic compounds made up of carbon, hydrogen, oxygen and nitrogen. It is the presence of the nitrogen which sets protein apart from other nutrients. Since we have no other source of nitrogen (being unable to absorb it from the air, like plants can), one of the most important roles of protein is to bring nitrogen into the body.
One of the primary uses of protein in the body is to synthesize structural proteins such as muscle, skin and hair. Protein is also used to synthesize peptide hormones such as growth hormone (GH), insulin-like growth factor 1 (IGF-1), insulin and glucagon. Additionally, protein is used to synthesize transport proteins, such as albumin, which is used to transport other substances through the bloodstream (1).
Proteins are made up of sub-units called amino acids (AAs). There are 20 AAs which occur in food, although more are present in the body (1). Two examples of non-dietary AAs are hydroxyproline, which is a by-product of the breakdown of connective tissue, and 3-methylhistidine, which is a by-product of the breakdown of muscle tissue.
Individual AAs are called peptides. When two AA’s are bonded together, it is called a di-peptide. Three AA’s bound together are called a tri-peptide, and four or more are called an oligo- or poly-peptide.
Indispensable and dispensable amino acids
The 20 dietary AAs are typically subdivided into two categories. In the past, these categories were termed essential and inessential (or non-essential). Essential AAs could not be synthesized in the body, and had to come from the diet, while inessential AAs could be made within the body.
Since all amino acids are essential, in that they are required for life, the categories have been renamed. The more accurate/modern terminology is dispensable AA (which can be made within the body) and indispensable AA (which must come from the diet).
However even this nomenclature is incomplete and is not sufficient to cover all possibilities. Depending on the metabolic state of the body, certain dispensable amino acids may become indispensable (2). For example, glutamine, which is normally considered a dispensable amino acid, may be required in such high quantities under certain conditions, that enough can not be made in the body, and it becomes indispensable (3). One of these situations is following trauma or surgery, where glutamine requirements go up significantly. In this case, glutamine is termed a conditionally indispensable AA.
Another example is cysteine, which reduces the requirements for methionine, and tyrosine, which reduces the requirements for tryptophan. Under situations where insufficient methionine or tryptophan are being consumed, cysteine and tyrosine would become indispensable (1). There are several other sub-categories which can also be delineated but they can be ignored for the purpose of this article (2).
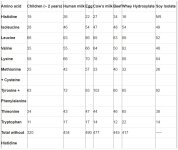
A list of dispensable and indispensable amino acids appears in table 1. (SEE IMAGE BELOW)
Complete and incomplete proteins
In the past, dietary proteins were classified as complete, meaning that all indispensable AAs were present ; or incomplete, meaning that one or more of the indispensable AAs was absent. However, with few exceptions (e.g. gelatin) every dietary protein contains all of the AAs in varying amounts. This means that the concept of ‘complete’ and ‘incomplete’ proteins is incorrect.
Since all proteins are complete, it is more accurate to refer to the limiting AA of a given protein, which is the indispensable AA occurring in the lowest quantity relative to what is required. One can also determine the second limiting AA, which is the indispensable AA occurring in the second least quantity, etc. The limiting AA will affect how well a given protein can be used by the body.
As an example, grains are typically very low in lysine but high in methionine while legumes are low in methionine but high in lysine. This complementarity between vegetable proteins led to the premise of combining grains and legumes together, to obtain a ‘complete’ protein (1). In general, limiting AAs should be a non-issue unless an individual is consuming all of their protein from a single source, and only if that source is a poor quality protein to begin with. That is, since proteins differ in their limiting AA, someone eating a variety of protein sources should fulfill their AA requirements with little difficulty. However, consuming large amounts of a low quality protein, one could consume adequate AAs for health and functioning. This would simply be an inefficient way of doing things because an excess of non-limiting AAs would be consumed simply to provide enough of the limiting AA.
Section 2: Protein digestion
Overview of protein digestion
Proteins are broken down in the stomach into smaller chains of AAs via various enzymes such as hydrochloric acid, trypsinogen and pepsinogen which break the bonds between AAs (1). In essence, these enzymes can be though of as scissors which cut large proteins into smaller chunks, which are then further digested. The specifics of breakdown are unimportant for this discussion and interested readers are referred to any biochemistry or nutrition textbook for details (1).
The breakdown of whole protein ultimately produces peptide chains which vary in length. This includes single AAs (peptides), chains of two AAs (di-peptides), and chains of three AAs (tri-peptides). Less than 5% of ingested protein is lost daily in the feces (1).
AAs are absorbed through the intestine via specific transporters. Depending on the transporter type, a certain AA will be absorbed and transported across the intestinal wall into the bloodstream. Additionally, many AA transporters carry more than one amino acid (1). This means that individuals who take large amounts of a single amino acid may overload a given transporter, and impair transport of a different amino acid which is carried by that same transporter. That is, like most nutrients, excess intake of one amino acid can potentially lead to deficiencies of another, due to competition for the same transport mechanism (1).
In addition to the single AA transporters, there are also transporters to carry di- and tri-peptides into the bloodstream (4). Chains longer than 4 AAs cannot be transported across the intestinal wall directly, and must be broken down to smaller chains prior to absorption (4).
The chemistry of protein digestion and transport have implications for a number of supplements. The first is the rash of ‘oral peptide hormones’ (such as GH or IGF-1) which are being marketed. Since peptide hormones are far longer than 4 AA in length, there is simply no way that oral ingestion can get any of the active hormone into the bloodstream. This assumes that such a product was real in the first place, which it is probably not since GH and IGF-1 are very expensive and are only available by prescription.
The protein digesting enzymes will break down orally ingested peptide hormones into smaller amino acid chains which will be treated just like any other dietary protein. Put differently, there’s a reason that GH, IGF-1 and insulin have to be injected: because they are broken down in the stomach to peptide chains, losing any value they might have as hormones.
The same holds for glandular supplements. For those who weren’t bodybuilding back in the 80’s, glandulars were dried extracts of various glands, which were supposed to improve the function of that same gland in the person who took it. So a thyroid glandular would consist of ground up thyroid gland (which is made of protein like most other tissues in the body) and ingestion was supposed to improve your thyroid function. A testicular (or orchic) glandular was ground up testicular tissue and was supposed to improve testicular function. As a large protein, any glandular will be simply broken down into smaller amino acid chains and treated like any other form of protein, making the claims for glandulars absurd.
Whole proteins, hydrosylates, and free form AAs….oh my
The three major sources of dietary protein available to individuals are whole proteins (food), partially digested proteins called hydrosylates (most protein powders) and free form AA’s (formulations containing single peptides). Arguments can be made for and against all three types of protein.
An extremely important point that should be understood is that once AA’s enter the bloodstream, they are indistinguishable from one another unless they have been radioactively labeled for research purposes. In fact, it is impossible to distinguish AAs from dietary protein from the AA’s present in the body already (5). Therefore, the amino acids derived from an egg will be treated no differently than from an amino acid capsule in terms of it’s physiological effects on the body. In this sense, there is no difference between whole, partially digested and free form proteins because they all eventually end up in the bloodstream and are treated the same.
However, there will be differences in the speed of absorption between the various sources of protein. Whole proteins will take longer to digest and be released into the bloodstream than a protein hydrosylate since the hydrosylate has already been partially broken down. This is the idea behind consuming a protein hydrosylate immediately after training: to try and get AAs to the recovering muscles as quickly as possible.
A possible advantage of free-form AAs is that one can tailor the formulation to contain specific quantities of each AA. However this assumes that one knows what the optimal amounts of each AA are in the first place. Additionally, di- and tri- peptides (generated from the breakdown of whole proteins or hydrosylates) show slightly better and faster uptake than free form AA; most likely due to the presence of transporters for di- and tri-peptides in the body (1,4). When the decreased efficiency of uptake of free-form AA is combined with their generally higher cost per gram of protein, free-form AA mixtures should be considered an ineffective form of protein supplement. However, there are some specific AAs, such as glutamine or the BCAAs, which may possibly be of benefit taken by themselves, but these will be discussed in part 3.
Fast vs. slow dietary proteins: the next big thing or much ado about nothing?
A recent paper (6) has kicked off an entirely new category/fad of protein nutrition and marketing, that of fast versus slow dietary proteins. This idea is conceptually similar to the glycemic index (GI) concept which is applied to carbohydrates, and represents the speed at which they digest and affect blood glucose and insulin levels.
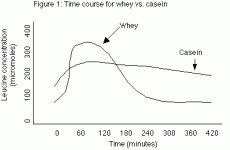
In this study, healthy subjects with a normal protein intake (16% of total calories) were fasted for 10 hours and then given either 30 grams of whey protein or 30 grams of casein (milk) protein. The primary finding of the study was that whey protein caused blood leucine levels (which are used as a marker of a variety of metabolic processes in the body) to increase rapidly, hitting a peak in 1 hour. However leucine levels decreased equally rapidly returning to normal by 4 hours. In contrast, casein caused a much slower rise in blood leucine levels, reaching a lower peak around 1 hour, but maintained that level for almost 7 hours (see figure 1). (SEE IMAGE BELOW)
Additionally, the researchers found that whey protein stimulated protein synthesis (which refers to the building of larger proteins from individual AAs) with no effect on protein breakdown (which refers to the breakdown of larger proteins to individual AAs), while the casein inhibited protein breakdown without affecting protein synthesis. Another observation was that whey protein increased leucine oxidation (burning) slightly more than the casein (31% vs. 24%), probably due to the faster rate of entry. Finally, leucine balance (determined as the amount ingested versus the amount stored) was higher for casein than whey. These observations lend themselves to multiple interpretations.
On the one hand, the effects on protein synthesis and breakdown are interesting and it appears that whey is an ‘anabolic’ protein while casein is ‘anti-catabolic’ protein, at least over a span of 7 hours. However, body leucine stores are also important for a variety of reasons (discussed in part 2 of this article) and it could be argued that casein is superior in that it led to net leucine retention by the body. I’m sure that how the study is interpreted will depend on whether the person who is interpreting it wants to sell whey, casein or a blend of the two.
This one study has already generated an article in the prestigious journal Nature (7) as well as in several bodybuilding magazines, with authors suggesting that whey and casein can be used to elicit differential physiological effects and spur growth. Protein supplements containing mixes of the so-called fast and slow proteins have also appeared on the market, with the idea being that one can get increased protein synthesis AND decreased protein breakdown, as well as keeping blood AA levels more stable.
To say that too much has been read into this single study would be putting it mildly. There are a number of issues which have been completely ignored by those reporting on this article which are discussed here. The first and perhaps most important point is that the subjects were fasted for 10 hours prior to being given the protein supplements. Protein synthesis and breakdown rates are very different after a 10 hour fast compared to the rates in the middle of the day after food has been eaten. After an overnight fast, muscle protein synthesis rates may be 50% lower than after food has been consumed (8). This means that any effects from a protein meal would be expected to be much higher first thing in the morning, versus a similar comparison done at a different time of the day.
Additionally, it is well known that the mixing of nutrients (i.e. carbohydrates and protein or carbohydrates, protein and fat) changes the absorption rate of nutrients into the bloodstream (1). Similarly, the presence of undigested food from a previous meal also affects digestion rate. All this study tells us is what will happen when whey or casein is taken by itself, after a 10 hour fast, on an empty stomach. To draw meaningful conclusions from this study to what might happen with the consumption of whey or casein with dietary fat (i.e. flax oil in the blender drink) or carbohydrates, or to other times of the day is impossible.
A final unanswered question is where the protein synthesized during the whey trial was stored (7). That is, the methodology of the study only told the researchers that protein was being synthesized and stored, not where it was going. This is, in fact, a major problem with most human protein research: it is generally difficult to know where stored protein is going unless a biopsy is taken. Since the goal of bodybuilders is to influence muscle protein synthesis, and not just increase whole body protein synthesis, it is important to know where the ingested protein is going. This topic will be discussed in greater detail in part 2 of this series.
Sufficed to say that it is just as reasonable to assume that it was being stored as liver protein as it is to assume it was being stored as muscle protein. Of course, saying that whey will increase liver protein synthesis won’t sell a lot of supplements. In part 2 of this series, the issues of protein synthesis and storage after a meal will be addressed in more detail, to help answer this question.
As a final comment, most serious bodybuilders eat a protein containing meal every 2-3 hours as it is. Since blood leucine didn’t drop until the 4 hour mark in the whey trial, is it going to make a huge difference whether a bodybuilder consumes whey or milk protein if they are eating every 3 hours? And if casein keeps blood leucine levels up for 7 hours, and whole proteins take even longer to fully digest, is it truly necessary to ingest protein every 3 hours during the day?
Some have suggested consuming a mix of whey and casein right before bedtime to get a sustained release of AA’s into the bloodstream and there may be some validity to this. Of course, any whole protein, combined with some carbs, fat and fiber would accomplish just as much.
A final question that this study raises has to do with the post-workout protein feeding. Consider that even the whey protein took 1 hour to raise blood leucine levels to it’s peak. If the idea is to provide AAs to a recovering muscle immediately after training, it might make more sense to consume protein an hour or two before the workout, so that AAs are hitting the bloodstream as the workout is ending.
Section 3: Protein requirements
There has been a long running debate over the protein requirements for athletes. On the one hand are dietitians and nutritionists, who continually argue that the Recommended Dietary Allowance (RDA) for protein is sufficient. On the other are the strength athletes and bodybuilders who have traditionally consumed a high protein diet in an effort to gain muscle mass more quickly. To address this controversy, we first have to look at the methodology used to determine protein requirements, and then look at basal requirements for protein. Then we can examine the effects of exercise on protein requirements.
Protein turnover and nitrogen balance
Every day your body is constantly breaking down some proteins, and synthesizing others. This is referred to as protein turnover and will be discussed in much greater detail in part 2 of this series (8). Under normal dietary conditions, an average person may turn over 300 grams of protein in a 24 hour period but obviously the body doesn’t require 300 grams of dietary protein per day. This is because most of the protein broken down is reused for protein synthesis.
However, no reactions in the body work with 100% efficiency and protein turnover is no different. In the process of protein turnover, some AAs will be oxidized, and nitrogen will be lost in the form of urea, creatinine, and other substances. Under normal protein intakes, as little as 4% of the total protein turnover may be lost (9). This can be altered with a high or low protein intake, a topic which is discussed in part 3 of this series (9).
Nitrogen is lost primarily in the urine, but some is lost in the feces, sweat, through the skin, finger nails, hair and other bodily excretions. Since it is tedious and difficult to measure all routes of nitrogen loss from the body (do you really want to measure fecal nitrogen losses) estimates are frequently used for fecal, skin, sweat, hair and nail losses (1).
Nitrogen balance compares the amount of nitrogen (from dietary protein) coming into the body versus what is being lost. If an individual is consuming more nitrogen than they are losing, they are in positive nitrogen balance, and are storing nitrogen in the body. If an individual is consuming the same amount of nitrogen as they are losing, they are simply in nitrogen balance, and are neither storing nor losing body nitrogen. If an individual is losing more nitrogen than they are consuming, they are in negative nitrogen balance and are losing body protein.
Since the breakdown of AAs are the main source of nitrogen loss, the excretion of nitrogen gives an indicator of AA breakdown. However nitrogen excretion does not tell you which amino acids are being broken down, or where they are coming from, so it is a crude measure at best.
Also, nitrogen balance depends heavily on total calorie intake (1). An individual who is fasting will lose more nitrogen than someone who is eating carbohydrates but is on a zero protein diet. Additionally, calories from dietary fat will not improve nitrogen balance as well as calories from dietary carbohydrate (10). Finally, individuals who consume very high protein intakes will also excrete more nitrogen, a topic discussed in part 2 of this series (9).
Obligatory protein requirements
The obligatory requirement for protein is defined as the amount necessary to equal what is being lost on a daily basis, so that a person remains in nitrogen balance. This is determined by measuring nitrogen excretion while an individual is on a protein-free diet. Since dietary nitrogen intake is zero, all excreted nitrogen is coming from the breakdown of body proteins. As mentioned above, this value assumes adequate dietary calories and a normal proportion of dietary carbohydrates.
The obligatory protein requirement has been estimated at 50-60 mg/kg/day (9,11). Thus a 100 kg (200 pound) person will lose 5-6 grams of nitrogen per day. Since protein is roughly 16% nitrogen, the loss of 5-6 grams of nitrogen is equivalent to the loss of 33 grams of protein per day.
To correct for digestibility issues, as well as individual variability, a ‘safety factor’ has been added to the above value (11). This is the basis of the RDA for protein which comes out to 0.8 g/kg/day or 0.36 g/lb/day, which should be sufficient protein for 95% of the population to maintain their current protein stores (11).
For the average male, this works out to roughly 55 grams of protein per day, for the average female 44 grams of protein per day, although this assumes that high quality protein and sufficient energy is being consumed (1). It should be noted that most Americans habitually consume two or three times this much protein on a daily basis, due to their high reliance on animal proteins (11).
In addition to protein requirements, the body has requirements for individual indispensable AAs. There is currently a great deal of debate over the AA requirements for humans at varying times of life (see for example 12,13). Since this requirement for indispensable AAs is tied intimately in with the issue of protein quality, a detailed discussion will have to wait until part 2.
Athletes and protein
Contrary to what many dietitians believe, the RDA was never meant to provide protein requirements for active individuals. In fact, the RDA handbook, which is the official guidelines handed down from the government regarding dietary needs, states “No added allowance is made here for the usual stresses encountered in daily living, which can give rise to transient increases in urinary nitrogen output. It is assumed that the subjects of experiments forming the basis for the requirement estimates are usually exposed to the same stresses as the population generally.” (11, pg. 71). As any bodybuilder knows, intense training falls outside the definition of ‘usual stresses encountered in daily living’. Additionally, numerous studies have shown that exercise increases the need for protein (for example 14,15).
Both aerobic exercise and strength training increase protein requirements although they do so for different reasons. During aerobic exercise, AAs can be used for energy production (especially the branch-chain AA’s, leucine, isoleucine, and valine) and may provide up to 10% of the total energy produced during long-duration activity (16). This occurs to a greater degree if glycogen is depleted (17) which is why excessive cardio may be even more catabolic on a low-carbohydrate diet.
While AAs do not contribute significantly to energy production during weight training, there is still net breakdown of AAs, as well as increased requirements for new protein synthesis.
Ultimately, the cause of the increased protein requirements is less important than the observation that protein requirements most definitely increase. Data reviewed by Dr. Peter Lemon indicates that endurance athletes may need 1.2-1.4 g/kg of protein (0.54-0.63 g/lb) and strength athletes 1.6-1.8 g/kg (0.72-0.8 g/lb) per pound to maintain a positive nitrogen balance (meaning that protein is being stored in the body) (16).
Although there is some limited research suggesting that even higher protein intakes may increase the rate of lean body mass gain (5,18), this has not been found in all studies. As will be discussed in part 2 of this article, excess protein tends to be oxidized (burned off).
The second part of this article will discuss the issue of protein quality, and will develop a simplified model of how protein can be used in the body. Part 3 will address the adaptations in protein metabolism which occur as a result of various conditions such as high and low protein intakes, high and low calorie intakes, and starvation.
BUY IRONMAG LABS COMPLETE PROTEIN
References:
1. “Advanced nutrition and human metabolism, 2nd ed.” James L. Groff, Sareen S. Gropper, Sara M. Hunt. West Publishing, 1995.
2. S.A. Laidlaw and J.D. Kopple “Newer concepts of the indispensable amino acids” Am J Clin Nutr (1987) 46: 593-605.
3. Lacey, J. and D. Wilmore “Is glutamine a conditionally essential amino acid” Nutr Rev (1990) 48: 297-309.
4.Silk, D.B.A. et. al. “Protein digestion and amino acid and peptide absorption” Proceedings of the Nutrition Society (1985) 44: 63-72.
5. Fern, E.B. et. al. “Effects of exaggerated amino acid and protein supply in man” Experientia (1991) 47: 168-172.
6. Boirie, Y. et. al. “Slow and fast dietary proteins differently modulate postprandial protein accretion” Proc Natl Acad Sci USA (1997) 94: 14930-14935.
7. Gema Fruhbeck “Slow and fast dietary proteins” Nature (1998) 391: 843-844.
8. J.C. Waterlow “Protein turnover with special reference to man” Q J Exp Phys (1984) 69: 409-438.
9. J.C. Waterlow “Metabolic adaptation to low intakes of energy and protien” Ann Rev Nutr (1986) 6: 495-526.
10. Richardson, D.P. et. al. “Quantitativ effect of an isoenergetic exchange of fat for carbohydrate on dietary protein utilization in healthy young men” Am J Clin Nutr (1979) 32: 2217-2226.
11. National Research Council “Recommended dietary allowances 10th ed” National Academy Press, 1989.
12. Vernon Young and P.L. Pellet “Protein evaluation, amino acid scoring and the food and drug administration’s proposed food labeling regulations” J Nutr (1990) 121: 145-150.
13. Joe Millward “Can we define indispensable amino acid requirements and assess protein quality in adults?” J Nutr (124) 1509S-1516S.
14. Lemon, PWR et. al. “Protein requirements and muscle mass/strength changes during intensive training in novice bodybuilders” J App Phys (1992) 73: 767-775.
15. Tarnopolsky, MA et. al. “Evaluation of protein requirements for trained strength athletes” J Appl Physiol (1992) 73: 1986-1995.
16. Peter Lemon “Is increased dietary protein necessary or beneficial for individuals with a physically active lifestyle” Nutr Rev (1996) 54: S169-S175.
17.Lemon, P.R. and J.P. Mullin “Effect of initial muscle glycogen level on protein catabolism during exercise” J Appl Physiol (1980) 48: 624-629.
18. Dragan, G.I. et. al. “Researches concerning the effects of Refit on elite weightlifters” J Sports Med (1985) 25: 246-250.
by Lyle McDonald
If you ask 10 bodybuilders what the most important nutrient is for putting on muscle, you will most likely get 10 identical answers (a rarity in sports nutrition). That answer is, of course: PROTEIN! Every bodybuilder knows that, no matter what else you do, if you aren’t getting enough protein, you won’t grow.
Unfortunately, the obsession that bodybuilders have with protein has made them susceptible to all kinds of marketing hype. Whether it involves protein intake, quality, type, form, etc., marketing types know how to push a bodybuilder’s buttons when it comes to protein.
If you want to sell a lot of protein powder, just throw around terms like ‘nitrogen balance/retention’, ‘biological value’ or ‘anti-catabolic’ and the money will start rolling in. If you want to scare bodybuilders into listening to you, start talking about amino acid oxidation (burning) with high protein intakes and how ‘horrible’ it is (too bad sarcasm doesn’t carry to the written word). Then move in for the kill and sell them your product.
Like most aspects of bodybuilding (and the supplement industry in general), the issue of protein is driven more by marketing hype than physiological reality. The purpose of this article series is to address some technical issues regarding protein and to clear up some of the major misconceptions that currently exist. Although some of the information is decidedly technical, I will try to avoid unnecessarily nasty details as much as possible.
This first part will deal primarily with some definitions and technical background, so that later information will make more sense. In part 2, protein quality as well as a simplified model of amino acid metabolism will be developed. This will help readers to understand the discussion of adaptation to both high and low protein intake, as well as varying calorie levels. Although a discussion of every amino acid is beyond the scope of this article series, specific amino acids such as glutamine, leucine and alanine will be discussed as necessary.
Section 1: Definitions and basic background
What is a protein?
Proteins are organic compounds made up of carbon, hydrogen, oxygen and nitrogen. It is the presence of the nitrogen which sets protein apart from other nutrients. Since we have no other source of nitrogen (being unable to absorb it from the air, like plants can), one of the most important roles of protein is to bring nitrogen into the body.
One of the primary uses of protein in the body is to synthesize structural proteins such as muscle, skin and hair. Protein is also used to synthesize peptide hormones such as growth hormone (GH), insulin-like growth factor 1 (IGF-1), insulin and glucagon. Additionally, protein is used to synthesize transport proteins, such as albumin, which is used to transport other substances through the bloodstream (1).
Proteins are made up of sub-units called amino acids (AAs). There are 20 AAs which occur in food, although more are present in the body (1). Two examples of non-dietary AAs are hydroxyproline, which is a by-product of the breakdown of connective tissue, and 3-methylhistidine, which is a by-product of the breakdown of muscle tissue.
Individual AAs are called peptides. When two AA’s are bonded together, it is called a di-peptide. Three AA’s bound together are called a tri-peptide, and four or more are called an oligo- or poly-peptide.
Indispensable and dispensable amino acids
The 20 dietary AAs are typically subdivided into two categories. In the past, these categories were termed essential and inessential (or non-essential). Essential AAs could not be synthesized in the body, and had to come from the diet, while inessential AAs could be made within the body.
Since all amino acids are essential, in that they are required for life, the categories have been renamed. The more accurate/modern terminology is dispensable AA (which can be made within the body) and indispensable AA (which must come from the diet).
However even this nomenclature is incomplete and is not sufficient to cover all possibilities. Depending on the metabolic state of the body, certain dispensable amino acids may become indispensable (2). For example, glutamine, which is normally considered a dispensable amino acid, may be required in such high quantities under certain conditions, that enough can not be made in the body, and it becomes indispensable (3). One of these situations is following trauma or surgery, where glutamine requirements go up significantly. In this case, glutamine is termed a conditionally indispensable AA.
Another example is cysteine, which reduces the requirements for methionine, and tyrosine, which reduces the requirements for tryptophan. Under situations where insufficient methionine or tryptophan are being consumed, cysteine and tyrosine would become indispensable (1). There are several other sub-categories which can also be delineated but they can be ignored for the purpose of this article (2).
A list of dispensable and indispensable amino acids appears in table 1. (SEE IMAGE BELOW)
Complete and incomplete proteins
In the past, dietary proteins were classified as complete, meaning that all indispensable AAs were present ; or incomplete, meaning that one or more of the indispensable AAs was absent. However, with few exceptions (e.g. gelatin) every dietary protein contains all of the AAs in varying amounts. This means that the concept of ‘complete’ and ‘incomplete’ proteins is incorrect.
Since all proteins are complete, it is more accurate to refer to the limiting AA of a given protein, which is the indispensable AA occurring in the lowest quantity relative to what is required. One can also determine the second limiting AA, which is the indispensable AA occurring in the second least quantity, etc. The limiting AA will affect how well a given protein can be used by the body.
As an example, grains are typically very low in lysine but high in methionine while legumes are low in methionine but high in lysine. This complementarity between vegetable proteins led to the premise of combining grains and legumes together, to obtain a ‘complete’ protein (1). In general, limiting AAs should be a non-issue unless an individual is consuming all of their protein from a single source, and only if that source is a poor quality protein to begin with. That is, since proteins differ in their limiting AA, someone eating a variety of protein sources should fulfill their AA requirements with little difficulty. However, consuming large amounts of a low quality protein, one could consume adequate AAs for health and functioning. This would simply be an inefficient way of doing things because an excess of non-limiting AAs would be consumed simply to provide enough of the limiting AA.
Section 2: Protein digestion
Overview of protein digestion
Proteins are broken down in the stomach into smaller chains of AAs via various enzymes such as hydrochloric acid, trypsinogen and pepsinogen which break the bonds between AAs (1). In essence, these enzymes can be though of as scissors which cut large proteins into smaller chunks, which are then further digested. The specifics of breakdown are unimportant for this discussion and interested readers are referred to any biochemistry or nutrition textbook for details (1).
The breakdown of whole protein ultimately produces peptide chains which vary in length. This includes single AAs (peptides), chains of two AAs (di-peptides), and chains of three AAs (tri-peptides). Less than 5% of ingested protein is lost daily in the feces (1).
AAs are absorbed through the intestine via specific transporters. Depending on the transporter type, a certain AA will be absorbed and transported across the intestinal wall into the bloodstream. Additionally, many AA transporters carry more than one amino acid (1). This means that individuals who take large amounts of a single amino acid may overload a given transporter, and impair transport of a different amino acid which is carried by that same transporter. That is, like most nutrients, excess intake of one amino acid can potentially lead to deficiencies of another, due to competition for the same transport mechanism (1).
In addition to the single AA transporters, there are also transporters to carry di- and tri-peptides into the bloodstream (4). Chains longer than 4 AAs cannot be transported across the intestinal wall directly, and must be broken down to smaller chains prior to absorption (4).
The chemistry of protein digestion and transport have implications for a number of supplements. The first is the rash of ‘oral peptide hormones’ (such as GH or IGF-1) which are being marketed. Since peptide hormones are far longer than 4 AA in length, there is simply no way that oral ingestion can get any of the active hormone into the bloodstream. This assumes that such a product was real in the first place, which it is probably not since GH and IGF-1 are very expensive and are only available by prescription.
The protein digesting enzymes will break down orally ingested peptide hormones into smaller amino acid chains which will be treated just like any other dietary protein. Put differently, there’s a reason that GH, IGF-1 and insulin have to be injected: because they are broken down in the stomach to peptide chains, losing any value they might have as hormones.
The same holds for glandular supplements. For those who weren’t bodybuilding back in the 80’s, glandulars were dried extracts of various glands, which were supposed to improve the function of that same gland in the person who took it. So a thyroid glandular would consist of ground up thyroid gland (which is made of protein like most other tissues in the body) and ingestion was supposed to improve your thyroid function. A testicular (or orchic) glandular was ground up testicular tissue and was supposed to improve testicular function. As a large protein, any glandular will be simply broken down into smaller amino acid chains and treated like any other form of protein, making the claims for glandulars absurd.
Whole proteins, hydrosylates, and free form AAs….oh my
The three major sources of dietary protein available to individuals are whole proteins (food), partially digested proteins called hydrosylates (most protein powders) and free form AA’s (formulations containing single peptides). Arguments can be made for and against all three types of protein.
An extremely important point that should be understood is that once AA’s enter the bloodstream, they are indistinguishable from one another unless they have been radioactively labeled for research purposes. In fact, it is impossible to distinguish AAs from dietary protein from the AA’s present in the body already (5). Therefore, the amino acids derived from an egg will be treated no differently than from an amino acid capsule in terms of it’s physiological effects on the body. In this sense, there is no difference between whole, partially digested and free form proteins because they all eventually end up in the bloodstream and are treated the same.
However, there will be differences in the speed of absorption between the various sources of protein. Whole proteins will take longer to digest and be released into the bloodstream than a protein hydrosylate since the hydrosylate has already been partially broken down. This is the idea behind consuming a protein hydrosylate immediately after training: to try and get AAs to the recovering muscles as quickly as possible.
A possible advantage of free-form AAs is that one can tailor the formulation to contain specific quantities of each AA. However this assumes that one knows what the optimal amounts of each AA are in the first place. Additionally, di- and tri- peptides (generated from the breakdown of whole proteins or hydrosylates) show slightly better and faster uptake than free form AA; most likely due to the presence of transporters for di- and tri-peptides in the body (1,4). When the decreased efficiency of uptake of free-form AA is combined with their generally higher cost per gram of protein, free-form AA mixtures should be considered an ineffective form of protein supplement. However, there are some specific AAs, such as glutamine or the BCAAs, which may possibly be of benefit taken by themselves, but these will be discussed in part 3.
Fast vs. slow dietary proteins: the next big thing or much ado about nothing?
A recent paper (6) has kicked off an entirely new category/fad of protein nutrition and marketing, that of fast versus slow dietary proteins. This idea is conceptually similar to the glycemic index (GI) concept which is applied to carbohydrates, and represents the speed at which they digest and affect blood glucose and insulin levels.
In this study, healthy subjects with a normal protein intake (16% of total calories) were fasted for 10 hours and then given either 30 grams of whey protein or 30 grams of casein (milk) protein. The primary finding of the study was that whey protein caused blood leucine levels (which are used as a marker of a variety of metabolic processes in the body) to increase rapidly, hitting a peak in 1 hour. However leucine levels decreased equally rapidly returning to normal by 4 hours. In contrast, casein caused a much slower rise in blood leucine levels, reaching a lower peak around 1 hour, but maintained that level for almost 7 hours (see figure 1). (SEE IMAGE BELOW)
Additionally, the researchers found that whey protein stimulated protein synthesis (which refers to the building of larger proteins from individual AAs) with no effect on protein breakdown (which refers to the breakdown of larger proteins to individual AAs), while the casein inhibited protein breakdown without affecting protein synthesis. Another observation was that whey protein increased leucine oxidation (burning) slightly more than the casein (31% vs. 24%), probably due to the faster rate of entry. Finally, leucine balance (determined as the amount ingested versus the amount stored) was higher for casein than whey. These observations lend themselves to multiple interpretations.
On the one hand, the effects on protein synthesis and breakdown are interesting and it appears that whey is an ‘anabolic’ protein while casein is ‘anti-catabolic’ protein, at least over a span of 7 hours. However, body leucine stores are also important for a variety of reasons (discussed in part 2 of this article) and it could be argued that casein is superior in that it led to net leucine retention by the body. I’m sure that how the study is interpreted will depend on whether the person who is interpreting it wants to sell whey, casein or a blend of the two.
This one study has already generated an article in the prestigious journal Nature (7) as well as in several bodybuilding magazines, with authors suggesting that whey and casein can be used to elicit differential physiological effects and spur growth. Protein supplements containing mixes of the so-called fast and slow proteins have also appeared on the market, with the idea being that one can get increased protein synthesis AND decreased protein breakdown, as well as keeping blood AA levels more stable.
To say that too much has been read into this single study would be putting it mildly. There are a number of issues which have been completely ignored by those reporting on this article which are discussed here. The first and perhaps most important point is that the subjects were fasted for 10 hours prior to being given the protein supplements. Protein synthesis and breakdown rates are very different after a 10 hour fast compared to the rates in the middle of the day after food has been eaten. After an overnight fast, muscle protein synthesis rates may be 50% lower than after food has been consumed (8). This means that any effects from a protein meal would be expected to be much higher first thing in the morning, versus a similar comparison done at a different time of the day.
Additionally, it is well known that the mixing of nutrients (i.e. carbohydrates and protein or carbohydrates, protein and fat) changes the absorption rate of nutrients into the bloodstream (1). Similarly, the presence of undigested food from a previous meal also affects digestion rate. All this study tells us is what will happen when whey or casein is taken by itself, after a 10 hour fast, on an empty stomach. To draw meaningful conclusions from this study to what might happen with the consumption of whey or casein with dietary fat (i.e. flax oil in the blender drink) or carbohydrates, or to other times of the day is impossible.
A final unanswered question is where the protein synthesized during the whey trial was stored (7). That is, the methodology of the study only told the researchers that protein was being synthesized and stored, not where it was going. This is, in fact, a major problem with most human protein research: it is generally difficult to know where stored protein is going unless a biopsy is taken. Since the goal of bodybuilders is to influence muscle protein synthesis, and not just increase whole body protein synthesis, it is important to know where the ingested protein is going. This topic will be discussed in greater detail in part 2 of this series.
Sufficed to say that it is just as reasonable to assume that it was being stored as liver protein as it is to assume it was being stored as muscle protein. Of course, saying that whey will increase liver protein synthesis won’t sell a lot of supplements. In part 2 of this series, the issues of protein synthesis and storage after a meal will be addressed in more detail, to help answer this question.
As a final comment, most serious bodybuilders eat a protein containing meal every 2-3 hours as it is. Since blood leucine didn’t drop until the 4 hour mark in the whey trial, is it going to make a huge difference whether a bodybuilder consumes whey or milk protein if they are eating every 3 hours? And if casein keeps blood leucine levels up for 7 hours, and whole proteins take even longer to fully digest, is it truly necessary to ingest protein every 3 hours during the day?
Some have suggested consuming a mix of whey and casein right before bedtime to get a sustained release of AA’s into the bloodstream and there may be some validity to this. Of course, any whole protein, combined with some carbs, fat and fiber would accomplish just as much.
A final question that this study raises has to do with the post-workout protein feeding. Consider that even the whey protein took 1 hour to raise blood leucine levels to it’s peak. If the idea is to provide AAs to a recovering muscle immediately after training, it might make more sense to consume protein an hour or two before the workout, so that AAs are hitting the bloodstream as the workout is ending.
Section 3: Protein requirements
There has been a long running debate over the protein requirements for athletes. On the one hand are dietitians and nutritionists, who continually argue that the Recommended Dietary Allowance (RDA) for protein is sufficient. On the other are the strength athletes and bodybuilders who have traditionally consumed a high protein diet in an effort to gain muscle mass more quickly. To address this controversy, we first have to look at the methodology used to determine protein requirements, and then look at basal requirements for protein. Then we can examine the effects of exercise on protein requirements.
Protein turnover and nitrogen balance
Every day your body is constantly breaking down some proteins, and synthesizing others. This is referred to as protein turnover and will be discussed in much greater detail in part 2 of this series (8). Under normal dietary conditions, an average person may turn over 300 grams of protein in a 24 hour period but obviously the body doesn’t require 300 grams of dietary protein per day. This is because most of the protein broken down is reused for protein synthesis.
However, no reactions in the body work with 100% efficiency and protein turnover is no different. In the process of protein turnover, some AAs will be oxidized, and nitrogen will be lost in the form of urea, creatinine, and other substances. Under normal protein intakes, as little as 4% of the total protein turnover may be lost (9). This can be altered with a high or low protein intake, a topic which is discussed in part 3 of this series (9).
Nitrogen is lost primarily in the urine, but some is lost in the feces, sweat, through the skin, finger nails, hair and other bodily excretions. Since it is tedious and difficult to measure all routes of nitrogen loss from the body (do you really want to measure fecal nitrogen losses) estimates are frequently used for fecal, skin, sweat, hair and nail losses (1).
Nitrogen balance compares the amount of nitrogen (from dietary protein) coming into the body versus what is being lost. If an individual is consuming more nitrogen than they are losing, they are in positive nitrogen balance, and are storing nitrogen in the body. If an individual is consuming the same amount of nitrogen as they are losing, they are simply in nitrogen balance, and are neither storing nor losing body nitrogen. If an individual is losing more nitrogen than they are consuming, they are in negative nitrogen balance and are losing body protein.
Since the breakdown of AAs are the main source of nitrogen loss, the excretion of nitrogen gives an indicator of AA breakdown. However nitrogen excretion does not tell you which amino acids are being broken down, or where they are coming from, so it is a crude measure at best.
Also, nitrogen balance depends heavily on total calorie intake (1). An individual who is fasting will lose more nitrogen than someone who is eating carbohydrates but is on a zero protein diet. Additionally, calories from dietary fat will not improve nitrogen balance as well as calories from dietary carbohydrate (10). Finally, individuals who consume very high protein intakes will also excrete more nitrogen, a topic discussed in part 2 of this series (9).
Obligatory protein requirements
The obligatory requirement for protein is defined as the amount necessary to equal what is being lost on a daily basis, so that a person remains in nitrogen balance. This is determined by measuring nitrogen excretion while an individual is on a protein-free diet. Since dietary nitrogen intake is zero, all excreted nitrogen is coming from the breakdown of body proteins. As mentioned above, this value assumes adequate dietary calories and a normal proportion of dietary carbohydrates.
The obligatory protein requirement has been estimated at 50-60 mg/kg/day (9,11). Thus a 100 kg (200 pound) person will lose 5-6 grams of nitrogen per day. Since protein is roughly 16% nitrogen, the loss of 5-6 grams of nitrogen is equivalent to the loss of 33 grams of protein per day.
To correct for digestibility issues, as well as individual variability, a ‘safety factor’ has been added to the above value (11). This is the basis of the RDA for protein which comes out to 0.8 g/kg/day or 0.36 g/lb/day, which should be sufficient protein for 95% of the population to maintain their current protein stores (11).
For the average male, this works out to roughly 55 grams of protein per day, for the average female 44 grams of protein per day, although this assumes that high quality protein and sufficient energy is being consumed (1). It should be noted that most Americans habitually consume two or three times this much protein on a daily basis, due to their high reliance on animal proteins (11).
In addition to protein requirements, the body has requirements for individual indispensable AAs. There is currently a great deal of debate over the AA requirements for humans at varying times of life (see for example 12,13). Since this requirement for indispensable AAs is tied intimately in with the issue of protein quality, a detailed discussion will have to wait until part 2.
Athletes and protein
Contrary to what many dietitians believe, the RDA was never meant to provide protein requirements for active individuals. In fact, the RDA handbook, which is the official guidelines handed down from the government regarding dietary needs, states “No added allowance is made here for the usual stresses encountered in daily living, which can give rise to transient increases in urinary nitrogen output. It is assumed that the subjects of experiments forming the basis for the requirement estimates are usually exposed to the same stresses as the population generally.” (11, pg. 71). As any bodybuilder knows, intense training falls outside the definition of ‘usual stresses encountered in daily living’. Additionally, numerous studies have shown that exercise increases the need for protein (for example 14,15).
Both aerobic exercise and strength training increase protein requirements although they do so for different reasons. During aerobic exercise, AAs can be used for energy production (especially the branch-chain AA’s, leucine, isoleucine, and valine) and may provide up to 10% of the total energy produced during long-duration activity (16). This occurs to a greater degree if glycogen is depleted (17) which is why excessive cardio may be even more catabolic on a low-carbohydrate diet.
While AAs do not contribute significantly to energy production during weight training, there is still net breakdown of AAs, as well as increased requirements for new protein synthesis.
Ultimately, the cause of the increased protein requirements is less important than the observation that protein requirements most definitely increase. Data reviewed by Dr. Peter Lemon indicates that endurance athletes may need 1.2-1.4 g/kg of protein (0.54-0.63 g/lb) and strength athletes 1.6-1.8 g/kg (0.72-0.8 g/lb) per pound to maintain a positive nitrogen balance (meaning that protein is being stored in the body) (16).
Although there is some limited research suggesting that even higher protein intakes may increase the rate of lean body mass gain (5,18), this has not been found in all studies. As will be discussed in part 2 of this article, excess protein tends to be oxidized (burned off).
The second part of this article will discuss the issue of protein quality, and will develop a simplified model of how protein can be used in the body. Part 3 will address the adaptations in protein metabolism which occur as a result of various conditions such as high and low protein intakes, high and low calorie intakes, and starvation.
BUY IRONMAG LABS COMPLETE PROTEIN
References:
1. “Advanced nutrition and human metabolism, 2nd ed.” James L. Groff, Sareen S. Gropper, Sara M. Hunt. West Publishing, 1995.
2. S.A. Laidlaw and J.D. Kopple “Newer concepts of the indispensable amino acids” Am J Clin Nutr (1987) 46: 593-605.
3. Lacey, J. and D. Wilmore “Is glutamine a conditionally essential amino acid” Nutr Rev (1990) 48: 297-309.
4.Silk, D.B.A. et. al. “Protein digestion and amino acid and peptide absorption” Proceedings of the Nutrition Society (1985) 44: 63-72.
5. Fern, E.B. et. al. “Effects of exaggerated amino acid and protein supply in man” Experientia (1991) 47: 168-172.
6. Boirie, Y. et. al. “Slow and fast dietary proteins differently modulate postprandial protein accretion” Proc Natl Acad Sci USA (1997) 94: 14930-14935.
7. Gema Fruhbeck “Slow and fast dietary proteins” Nature (1998) 391: 843-844.
8. J.C. Waterlow “Protein turnover with special reference to man” Q J Exp Phys (1984) 69: 409-438.
9. J.C. Waterlow “Metabolic adaptation to low intakes of energy and protien” Ann Rev Nutr (1986) 6: 495-526.
10. Richardson, D.P. et. al. “Quantitativ effect of an isoenergetic exchange of fat for carbohydrate on dietary protein utilization in healthy young men” Am J Clin Nutr (1979) 32: 2217-2226.
11. National Research Council “Recommended dietary allowances 10th ed” National Academy Press, 1989.
12. Vernon Young and P.L. Pellet “Protein evaluation, amino acid scoring and the food and drug administration’s proposed food labeling regulations” J Nutr (1990) 121: 145-150.
13. Joe Millward “Can we define indispensable amino acid requirements and assess protein quality in adults?” J Nutr (124) 1509S-1516S.
14. Lemon, PWR et. al. “Protein requirements and muscle mass/strength changes during intensive training in novice bodybuilders” J App Phys (1992) 73: 767-775.
15. Tarnopolsky, MA et. al. “Evaluation of protein requirements for trained strength athletes” J Appl Physiol (1992) 73: 1986-1995.
16. Peter Lemon “Is increased dietary protein necessary or beneficial for individuals with a physically active lifestyle” Nutr Rev (1996) 54: S169-S175.
17.Lemon, P.R. and J.P. Mullin “Effect of initial muscle glycogen level on protein catabolism during exercise” J Appl Physiol (1980) 48: 624-629.
18. Dragan, G.I. et. al. “Researches concerning the effects of Refit on elite weightlifters” J Sports Med (1985) 25: 246-250.